Text by Henrylito D. Tacio
Photos: UN
Have you ever wondered why that guileless-looking hair spray was a subject of controversy and was later banned in the late Seventies? And did you know that increased risk of cancer and eye cataracts are linked to this hapless cosmetic? If you haven’t, chances are, you also haven’t heard of ozone and the atmospheric ozone layer.
Until now, only a very few know the importance of the ozone layer. It is a stratospheric or upper atmospheric layer of air above the earth. It is a form of oxygen, and its molecule is made up of three oxygen atoms instead of two.
Scientists say ozone forms at an altitude between 6 and 30 miles above the earth when high-energy ultraviolet light from sunlight strikes oxygen molecules, splitting some of the normally two-atom oxygen molecules apart. Free oxygen atoms attach to other oxygen molecules, making ozone.
Although at sea level’s atmospheric pressure, the ozone would only be a few millimeters thick, it helps life on earth survive. The ozone layer acts as a shield against the deleterious radiation of the ultraviolet rays of the sun while allowing visible light to come through. This visible light is needed for the process of photosynthesis which helps plants to grow to give human beings food.
Aside from sheltering the earth from harmful radiation, the ozone layer also regulates the temperatures of our planet’s surface and atmosphere. It is for this reason that when the ozone in the stratosphere decreases, the unabsorbed radiation pierces the thin veil of the ozone and thus reaches the earth unfiltered. This makes the earth’s temperature warmer.
Studies have shown that exposure to large amounts of ultraviolet radiation have been associated with increasing risks of skin cancer and eye damage in human beings and changes in the immune systems of animals. Likewise, production yields in agriculture, marine, and natural resources have dramatically plunged to low levels due to the exposure.
Unfortunately, this protective ozone layer has been seriously threatened by some scientific inventions and also by the way we live in modern times.
In the early 1970s, scientists warned that the ozone layer was getting dangerously thin. In 1974, two American scientists – Mario Molina and F. Sherwood Rowland from the Jet Propulsion Institute of Pasadena and the University of Southern California in Irvine, respectively – contended that man-made chlorofluorocarbons (CFCs) were escaping into the atmosphere and “eating” the ozone layer.
However, policymakers largely ignored their assertions until 1985, when a team of British atmospheric chemists headed by Dr. Joseph Farman reported that during the previous eight years, a “hole” – as big as the United States and as deep as Mount Everest – had formed every September and October in the ozone layer over the Antarctic.
Scientists who are studying the hole in the ozone layer cited two major man-made chemicals: the chlorine compound, particularly chlorofluorocarbons (CFCs), and the bromine compounds, halons.
Developed in 1930, CFCs are used in refrigerators, air conditioners, and aerosol spray cans. They are also present in foam to make seat cushions and furniture stuffing. CFCs are utilized as a cleaning fluid as well as for delicate equipment like electronic machines and for dry-cleaning clothes.
Halons, on the other hand, are mostly used in fire extinguishing systems. They are said to be more dangerous than CFCs because halons are four to sixteen times more destructive once they reach the ozone layer!
Another ODS that is not given much attention is methyl bromide, the second most widely used pesticide in the world. Between 80 to 100 percent of the methyl bromide is subsequently released into the atmosphere, according to the environmental pressure group Friends of the Earth.
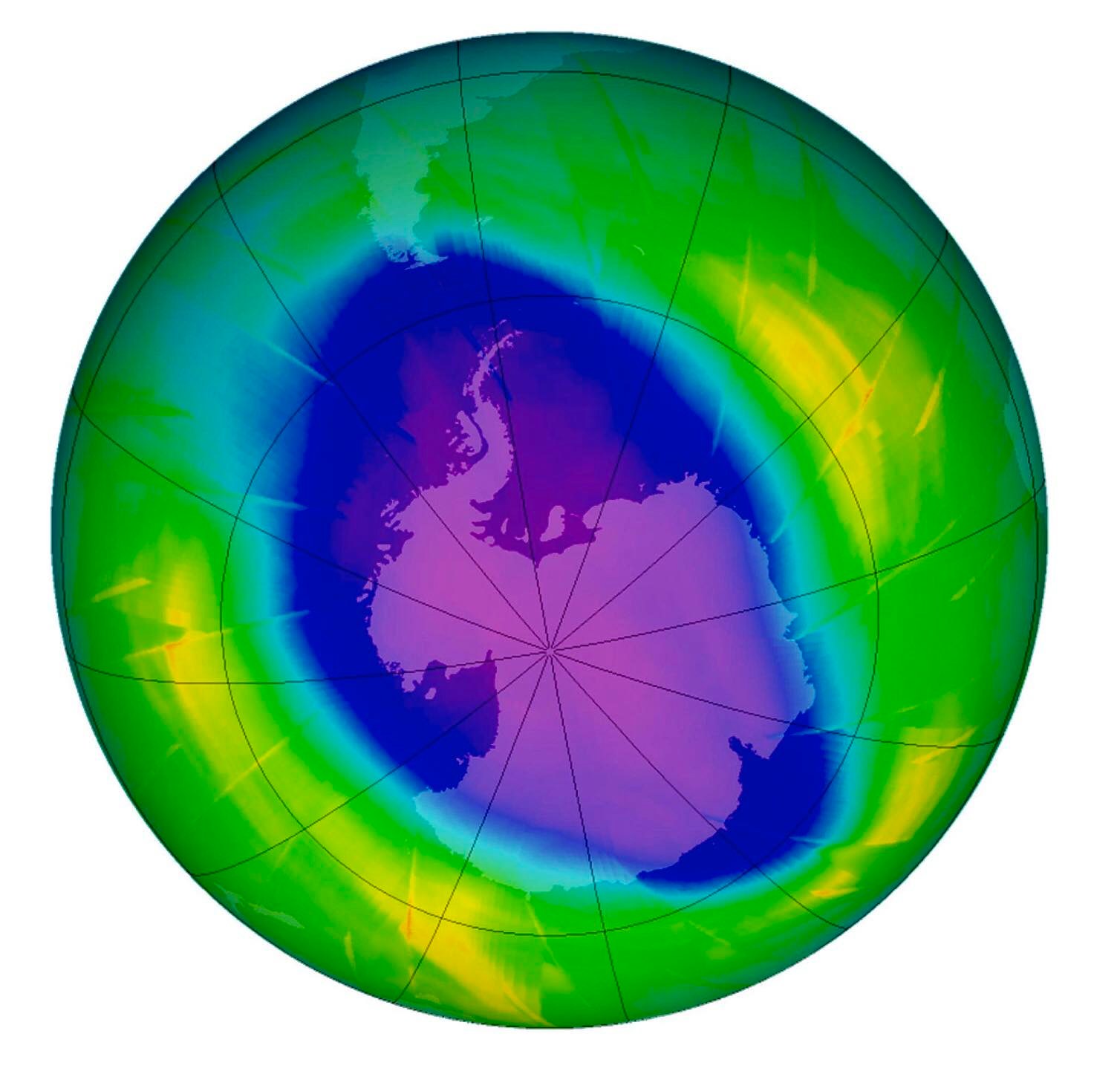
Natural phenomena like weather patterns and volcanic eruptions likewise contribute to the destruction of the ozone layer. Studies made by scientists of the European Arctic Stratospheric Experiment have shown that the June 1991 eruption of Mt. Pinatubo threatened the ozone layer. Large amounts of volcanic aerosol containing sulphur dioxide gas have been injected into the stratosphere, forming sulphuric acid.
Planes are also harmful to the ozone layer, experts claim. “When you have supersonic flights high in the stratosphere putting out nitrogen oxide then that would certainly be a dominant factor in ozone depletion,” explained Dr. John Pyle, a key atmospheric researcher for Britain’s leading environmental research body, the Natural Environment Research Council.
In mid-September 1987, 24 countries met in Montreal, Canada, and came up with a landmark effort to address ozone depletion. Called the Montreal Protocol, it was signed by the Philippines on September 14, 1988, and ratified on March 21, 1991.
“The Montreal Protocol has 197 state signatories, and I can say that the Montreal Protocol owes its success to countries like the Philippines, that for three decades, has been consistently cooperative and compliant to the targets and schedules it set to phase out ODS around the world,” said former Environment Secretary Roy A. Cimatu.
Now, the good news. Recent reports said that the ozone layer is slowly recovering from depletion. In 2016, ABC science reporter Dani Cooper reported that the ozone hole over Antarctica was “healing” almost 30 years after the world banned the chemicals responsible for its creation.
“The ozone hole above the Antarctic is now smaller than it was around the year 2000, by about 4 million square kilometers,” said renowned ozone hole expert Professor Susan Solomon of Massachusetts Institute of Technology.
She added, however, that the hole still averages about 17 million square kilometers in size. “It isn’t completely healed,” she pointed out, “but it’s better than the 21 million we had around 2000.”

