Scientists from the University of the Philippines – Diliman College of Science (UPD-CS) have simplified the process of making microscopic composite flowers that can neutralize the highly carcinogenic azo dyes widely used in food, clothes, and medicines.
Azo dyes are synthetic colorants that come in a variety of vivid colors, including red, orange, and yellow. They were commonly used in everything from denim and leather to soft drinks and jams. However, it was discovered that some azo dyes are closely linked to bladder cancer. Moreover, the regulated use and safe disposal of these carcinogenic azo dyes remain a global concern.
A new material that may help to safely degrade azo dyes was recently investigated by Enrico Daniel R. Legaspi, Prof. Michelle D. Regulacio, and Leila Andrea E. Pineda from the Institute of Chemistry (UPD-CS IC); Luce Vida A. Sayson of the Material Science and Engineering Program (UPD-CS MSEP); and colleagues from Singapore’s Agency for Science, Technology, and Research (A*STAR).
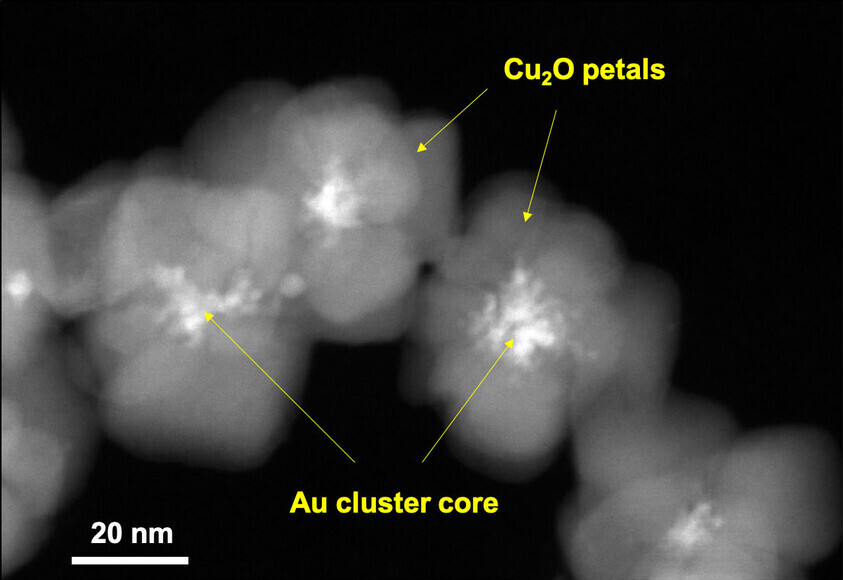
The nanocomposite material exhibits a flower-like structure, each just around 50 nanometers in diameter or less than half the width of a human hair, with a gold (Au) center surrounded by petal-like copper oxide (Cu2O) crystals. It was found that this configuration greatly enhances Cu2O’s ability to catalyze the breakdown of azo dyes into harmless chemicals.
The researchers said that this is the first time that this flower-like configuration has been synthesized in a single manufacturing setup, thereby paving the way for easier and more affordable production.
“The one-pot synthesis protocol presented in this work is a more straightforward and less laborious approach that does not require a separate pre-synthesis step. Furthermore, the synthesis can be conveniently performed at ambient conditions using nontoxic reagents,” the researchers explained in their paper.
“The uniquely designed Au-Cu2O nanoflowers were found to effectively catalyze the borohydride-mediated degradation of synthetic azo dyes. The hybrid exhibited superior catalytic activity relative to pristine Cu2O, underscoring the significance of creating a nanocomposite,” they added. (PR)
PHOTO CAPTION: Seen under an electron microscope, these nanoflowers—each one less than half the width of a human hair—feature a gold center surrounded by ‘petals’ made from a copper compound. This nanomaterial has been found to facilitate the degradation of widely used but highly toxic azo dyes. (Photo credit: Wiley-VCH)